A zeolite is a naturally occurring insoluble mineral of the sodium aluminosilicate
type. When hard water passes through a bed of small particles of such a mineral,
an ion exchange reaction takes place (Scheme 8.10). As more and more alkaline
earth metal ions are retained by the zeolite, its exchange capacity gradually
decreases. Regeneration of the sodium salt of the zeolite involves passing a
concentrated solution of NaCl through the zeolite. The calcium and magnesium
ions are displaced and leave with the solution (the reverse of Scheme 8.10). After
rinsing with water to eliminate the excess salt, the zeolite is ready for another cycle
of softening.
Many zeolites occur in nature but they can also be manufactured. The removal
of the alkaline earth metal ions is more effective the greater the surface area of the zeolite particles in contact with the water. For this reason, softening involves
percolation of the water down a packed column of the finely ground zeolite,
followed by periodic regeneration. Although the calcium and magnesium ions in
the water are replaced by sodium ions, these are relatively harmless in textile
processing.The chemical structures of aluminosilicates are based on the structure of silica.This consists of a three dimensional network of SiO4 units, in which the oxygen atoms have a tetrahedral arrangement around the central silicon atom. These
tetrahedra may have common corners or faces. In an aluminosilicate, a number of
aluminium atoms replace silicon atoms in the silica structure. The aluminium
atoms are bonded to four tetrahedral oxygen atoms but because their atomic
number is one less than silicon, each aluminium atom introduced has a negative
charge, balanced by incorporation of a cation such as Na+ or K+. It is these
cations that are available for exchange.
The newer synthetic polymer ion exchangers are much more versatile than the
zeolites and are widely used for water softening and demineralisation. They are
often called ion exchange resins. Many are based on polystyrene that has been
partly crosslinked by incorporation of a small amount of divinylbenzene (2 – 10%).
Suspension polymerisation of the styrene and divinylbenzene produces the
crosslinked polymer in the form of small beads. These have the appearance of a
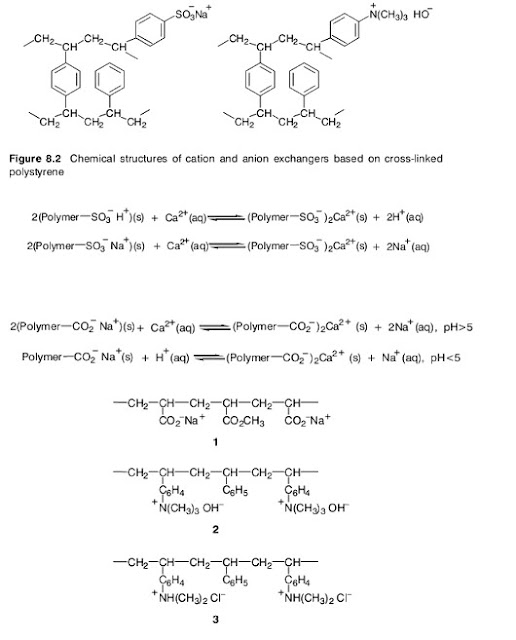
fine white sand. Sulphonation of some of the benzene rings, mainly on the bead
surfaces, provides anionic sites suitable for cation exchange (Figure 8.2). Since a
sulphonic acid is comparable in strength to a mineral acid, these are called strong
acid exchangers. They may absorb cations and release hydrogen ions, or, in the
form of their sodium salts, exchange sodium ions for other cations in the water
Weak acid cation exchanger, containing acrylic acid (ethenoic acid) units ( 1 );
strong basic anion exchange resin, containing quaternary ammonium hydroxide groups
(2 );weakly basic anion exchange resin with protonated secondary amino groups (3 )
The so-called weak acid cation exchangers are usually polymers containing
carboxylic acid groups, such as vinyl polymers containing acrylic acid (ethenoic
acid) units (1, in Figure 8.3). These are used in the form of their sodium salts.
They do not exchange hydrogen ions for cations below pH 5 since the free
carboxylic acid groups are barely dissociated at low pH values (Scheme 8.12).
Other types of ion exchange resins are available for anion exchange. These may
be of the strong basic type, containing quaternary ammonium hydroxide groups
(see Figure 8.2, and 2 in Figure 8.3), or weakly basic with protonated secondary
amino groups (3 , in Figure 8.3). Either type will have associated counter anions
that exchange with anions in the solution in contact with the resin. The weakly
basic types have ammonium ion groups that can lose a proton in contact with an
alkaline solution and therefore are ineffective above pH 10 (Scheme 8.13). The
ion exchange processes are again reversible so resin regeneration simply involves
treatment with a concentrated solution of a salt containing the appropriate anion.
Therefore, a strongly basic quaternary ammonium ion resin is regenerated using
NaOH solution, and a secondary amine type with NaCl or HCl solution.
The two most important properties of an ion exchanger are its capacity and its
selectivity. The capacity is the number of ions that a given mass of resin is capable
of binding by exchange, normally expressed in milliequivalents per gram of dry or
of wet resin. For example, 1.0 mmol of Na+ is the same as 1.0 mequiv, but 1.0 mmol of Ca2+ is 2.0 mequiv. Therefore, a resin with a capacity of 15.0 mequiv g– 1 would be capable of binding 15.0 mmol g– 1 Na+ or 7.5 mmol g–1 Ca2+. The selectivity of the resin determines how strongly it binds a given ion and therefore its ease of exchange. Clearly, for water softening a cation exchange resin should have a higher selectivity for Ca2+ and Mg2+ than for H+ or Na+. Fortunately, the selectivity is often greater for ions of higher ionic charge.
Besides these two properties, the degree of swelling of the resin in contact with
the water must be limited. The higher the degree of crosslinking, for example from
incorporation of more divinylbenzene in the polystyrene, the lower the extent of
swelling. It is also important that the resin particles have a large surface area and
that water is able to penetrate into the surface pores.
Water can be totally demineralised by firstly exchanging all cations using a
strongly acid form of a cation exchanger. Thus, a solution of salts M+X– becomes a
solution of acid H+X–, the M+ ions being retained by the resin. Subsequent
percolation through a packing of a strongly basic form of an anion exchanger
absorbs the X– ions and liberates HO– ions into the water. These then neutralise the H+ ions from the first step. The result is retention of all anions and cations
and the neutralisation of H+ and HO– to form water (Scheme 8.14). Thus, the
water has been demineralised. It may, however, still contain organic material and
dissolved carbon dioxide from the reaction of carbonate and bicarbonate with the
acid from the resin. A thorough aeration eliminates the carbon dioxide.
Demineralisation is important for water fed to very high pressure boilers.
The use of ion exchange resins for water treatment is relatively simple. The
resin is packed into a column containing water and treatment simply involves
flowing water up or down the column. The capacity of the resin and the ionic
content of the water determine when regeneration will be required. One problem
with beds of ion exchangers is the retention in the column of suspended matter
and living organisms in the water. Countercurrent rinsing and occasional
treatment with a bactericide minimise these problems. For removal of both cations
and anions (demineralisation), two columns in series are used, the first for strong
acid exchange and the second for strong base exchange. It is even possible to mix
anion and cation exchangers in the same bed. If the different types of particles
have different densities, they can be separated by sedimentation in a counterflow
of water, regenerated separately, and then re-mixed. Figure 8.4 shows a typical
series of processes for water softening.




we are manufacture, exporter and suppliers of ion exchange in india since 1954 with a range of mass transfer equipment.
ReplyDeleteThankfulness to my dad who informed me relating to this blog, this website is really amazing. Softeningwater
ReplyDeleteThis is great advice! Very honest and practical.I really enjoyed this post.
ReplyDeletedifference between zeolite and ion exchange process