Textile Solutions
Saturday, May 5, 2012
Waste water treatment
calculating water hardness
determination of water hardness
measuring water hardness
water hardness map
water hardness definition
water hardness test kit
aquarium water hardness
water softener
calculating water hardness
determination of water hardness
measuring water hardness
water hardness map
water hardness definition
water hardness test kit
aquarium water hardness
water softener
calculating water hardness
determination of water hardness
measuring water hardness
water hardness map
water hardness definition
water hardness test kit
aquarium water hardness
water softener
calculating water hardness
determination of water hardness
measuring water hardness
water hardness map
water hardness definition
water hardness test kit
aquarium water hardness
water softenercalculating water hardness
determination of water hardness
measuring water hardness
water hardness map
water hardness definition
water hardness test kit
aquarium water hardness
water softenercalculating water hardness
determination of water hardness
measuring water hardness
water hardness map
water hardness definition
water hardness test kit
aquarium water hardness
water softener
Water hardness in different terms
Water Hardness, Water treatment, Water hardness, Hardness of water, Water hardness measurement, Water softening method, Water Hardness, Water Hardness Water Hardness, Water treatment, Water hardness, Hardness of water, Water hardness measurement, Water softening method, Water Hardness, Water Hardness
Water Hardness, Water treatment, Water hardness, Hardness of water, Water hardness measurement, Water softening method, Water Hardness, Water Hardness
Water Hardness, Water treatment, Water hardness, Hardness of water, Water hardness measurement, Water softening method, Water Hardness, Water Hardness
Water Hardness, Water treatment, Water hardness, Hardness of water, Water hardness measurement, Water softening method, Water Hardness, Water Hardness
Water Hardness, Water treatment, Water hardness, Hardness of water, Water hardness measurement, Water softening method, Water Hardness, Water Hardness
Water softening by ion exchange method, Ion exchange method,exchange method, Ion exchange method,exchange method, Ion exchange method,exchange method, Ion exchange method,exchange method, Ion exchange method,exchange method, Ion exchange method,Water Hardness, Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Water treatment plant, Water treatment plant,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,Effluent treatment,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process,The lime exchange process, Minimum hardness in textile dyehouse.
Explanation of water hardness,Explanation of water hardness,Explanation of water hardness,Explanation of water hardness,Explanation of water hardness,Explanation of water hardness,Explanation of water hardness,
Water quality, water quality, water quality,Water quality, water quality, water quality,Water quality, water quality, water quality,Water quality, water quality, water quality,waste water treatment.
Monday, April 30, 2012
Effluent treatment
Any effluent treatment programme must consider whether the water
contaminants are biodegradable, over what time scale, and – if persistent – how
they will influence conditions down stream. Even the influence of subsequent
water chlorination for drinking purposes on the potential formation of toxic
organochlorine compounds must be considered. This a complex subject requiring
detailed on-site analysis before arriving at specific recommendations and their
implementation.
One very important strategy in reducing water pollution from a textile finishing
plant involves minimising waste and optimising process methods so that fewer
contaminants are discharged in the effluent. The following list gives some
examples of this.
(1) re-use of excess dye solution remaining at the end of a process – this can be incorporated into other dyeing recipes;
(2) mixing acidic and alkaline effluents before discharge to avoid excessive acidity or alkalinity;
(3) replacing starch-based sizing materials with polyvinyl alcohol, which can be recovered by membrane separation techniques and recycled;
(4) process changes and optimisation of the use of problematic chemicals – for example, for the oxidation of leuco vat dyes, hydrogen peroxide or sodium perborate can be substituted for sodium dichromate. Replacement of dyes that require aftertreatment with chromium or copper salts will have a beneficial impact on the immediate water environment. Glucose can replace sodium sulphide as a reducing agent for sulphur dyes. The dye manufacturers now offer ‘greener’ products and processes such as low salt/high fixation reactive dyes. Many companies are examining the possibilities of recycling water and chemicals, and of recovering heat from hot effluent. Various types of processes are used in textile effluent treatment. Dyehouse effluent has a composition that is highly time-dependent in terms of both the types and quantities of contaminants. A first stage in treatment is often an equalising lagoon. Equalisation involves holding the combined process effluents for a given period to allow stabilisation of pH and BOD, and time for sedimentation of some solids. This considerably reduces fluctuations in the composition of the water leaving the dyehouse, which can upset down stream processes such as activated sludge treatment. Biological treatment with organisms may be aerobic (with oxygen) or anaerobic (without oxygen). Many textile mills in urban areas discharge their effluent into municipal sewers. The sewage may be treated in an activated sludge plant. The effluent is mixed with micro-organisms, aerated and then the sludge allowed to settle. The phosphate and nitrogen nutrients needed for bacterial growth are not a problem if the industrial effluent is mixed with regular sewage. Activated sludge treatment considerably reduces the BOD by aerobic oxidation, and adsorption or coagulation of the contaminants. The effects are improved if the sludge is combined with activated carbon. This also protects the micro-organisms from heavy metals. They are sensitive to sudden changes in conditions and industrial effluents in the sewer system should be of relatively constant composition and concentration. The main problem is that of sludge disposal. After suitable treatment, it can be used as fertiliser, or for landfill. In a trickle filter plant, the effluent percolates through a filter bed with the bacteria growing on the surface of the filter medium. These aerobic microbial oxidation processes reduce the BOD, COD and TOC. The effect on colour from dyes, however, is often only marginal. Some dyes are adsorbed to some extent by the biological treatment. This has only limited effects in removing hydrolysed reactive dyes from the effluent. Because of the high levels of waste, textile auxiliary products should ideally biodegrade rapidly in water, although this is often not the case. Alkyl benzene sulphonate detergents, with a branched alkyl chain, such as that derived from propene tetramer, caused mountains of foam on rivers throughout the industrialised world in the 1960s because of their low rate of biodegradation. One of the most common effluent treatment methods is that of precipitation. This often involves a combination of precipitation of insoluble salts, coagulation of colloidal material and flocculation. This is similar in principle to the method used for clarifying water described in Section 8.1. Addition of lime (CaO) to the effluent is quite common. This neutralises any excess acidity and precipitates many types of anionic compounds. Treatments with alum or ferric chloride are also popular. The aluminium or ferric hydroxide, along with precipitated aluminium or ferric salts, removes colloidal matter and a number of anionic dyes. This reduces the COD, colour and suspended solids. Sedimentation is assisted by adding a flocculant such as a polyacrylic acid derivative, or a cationic polymer, the latter being able to bind hydrolysed reactive dyes. Again, the disposal of the sediment sludge needs to be considered. There are a variety of chemical technologies for effluent treatment. Few of these are used in the textile industry because of their cost. This situation may change as environmental protection becomes even more socially and politically acceptable. Chlorination with sodium hypochlorite and acid eliminates much organic material in waste water but may generate even more toxic organochlorine compounds. Oxidation by ozone is much safer but the cost of generating ozone by electrical discharge through oxygen gas is still prohibitive. Other technologies involve reverse osmosis and membrane filtration, adsorption on active carbon, or generation of coagulants by electrochemical techniques. The highest standards of effluent treatment require combinations of different types of treatment. These will probably become more significant, despite the expense, as regulatory controls are increasingly enforced.
(1) re-use of excess dye solution remaining at the end of a process – this can be incorporated into other dyeing recipes;
(2) mixing acidic and alkaline effluents before discharge to avoid excessive acidity or alkalinity;
(3) replacing starch-based sizing materials with polyvinyl alcohol, which can be recovered by membrane separation techniques and recycled;
(4) process changes and optimisation of the use of problematic chemicals – for example, for the oxidation of leuco vat dyes, hydrogen peroxide or sodium perborate can be substituted for sodium dichromate. Replacement of dyes that require aftertreatment with chromium or copper salts will have a beneficial impact on the immediate water environment. Glucose can replace sodium sulphide as a reducing agent for sulphur dyes. The dye manufacturers now offer ‘greener’ products and processes such as low salt/high fixation reactive dyes. Many companies are examining the possibilities of recycling water and chemicals, and of recovering heat from hot effluent. Various types of processes are used in textile effluent treatment. Dyehouse effluent has a composition that is highly time-dependent in terms of both the types and quantities of contaminants. A first stage in treatment is often an equalising lagoon. Equalisation involves holding the combined process effluents for a given period to allow stabilisation of pH and BOD, and time for sedimentation of some solids. This considerably reduces fluctuations in the composition of the water leaving the dyehouse, which can upset down stream processes such as activated sludge treatment. Biological treatment with organisms may be aerobic (with oxygen) or anaerobic (without oxygen). Many textile mills in urban areas discharge their effluent into municipal sewers. The sewage may be treated in an activated sludge plant. The effluent is mixed with micro-organisms, aerated and then the sludge allowed to settle. The phosphate and nitrogen nutrients needed for bacterial growth are not a problem if the industrial effluent is mixed with regular sewage. Activated sludge treatment considerably reduces the BOD by aerobic oxidation, and adsorption or coagulation of the contaminants. The effects are improved if the sludge is combined with activated carbon. This also protects the micro-organisms from heavy metals. They are sensitive to sudden changes in conditions and industrial effluents in the sewer system should be of relatively constant composition and concentration. The main problem is that of sludge disposal. After suitable treatment, it can be used as fertiliser, or for landfill. In a trickle filter plant, the effluent percolates through a filter bed with the bacteria growing on the surface of the filter medium. These aerobic microbial oxidation processes reduce the BOD, COD and TOC. The effect on colour from dyes, however, is often only marginal. Some dyes are adsorbed to some extent by the biological treatment. This has only limited effects in removing hydrolysed reactive dyes from the effluent. Because of the high levels of waste, textile auxiliary products should ideally biodegrade rapidly in water, although this is often not the case. Alkyl benzene sulphonate detergents, with a branched alkyl chain, such as that derived from propene tetramer, caused mountains of foam on rivers throughout the industrialised world in the 1960s because of their low rate of biodegradation. One of the most common effluent treatment methods is that of precipitation. This often involves a combination of precipitation of insoluble salts, coagulation of colloidal material and flocculation. This is similar in principle to the method used for clarifying water described in Section 8.1. Addition of lime (CaO) to the effluent is quite common. This neutralises any excess acidity and precipitates many types of anionic compounds. Treatments with alum or ferric chloride are also popular. The aluminium or ferric hydroxide, along with precipitated aluminium or ferric salts, removes colloidal matter and a number of anionic dyes. This reduces the COD, colour and suspended solids. Sedimentation is assisted by adding a flocculant such as a polyacrylic acid derivative, or a cationic polymer, the latter being able to bind hydrolysed reactive dyes. Again, the disposal of the sediment sludge needs to be considered. There are a variety of chemical technologies for effluent treatment. Few of these are used in the textile industry because of their cost. This situation may change as environmental protection becomes even more socially and politically acceptable. Chlorination with sodium hypochlorite and acid eliminates much organic material in waste water but may generate even more toxic organochlorine compounds. Oxidation by ozone is much safer but the cost of generating ozone by electrical discharge through oxygen gas is still prohibitive. Other technologies involve reverse osmosis and membrane filtration, adsorption on active carbon, or generation of coagulants by electrochemical techniques. The highest standards of effluent treatment require combinations of different types of treatment. These will probably become more significant, despite the expense, as regulatory controls are increasingly enforced.
DYEHOUSE EFFLUENT AND ITS TREATMENT
Contaminants in dyehouse effluents
For many years, the textile dyeing industry was a major source of water pollution.
Increasing public awareness of this problem has resulted in much stricter
legislation to protect the environment with controls on the types of contaminants
and the amounts released. Governments have also become much more aggressive
in updating legislation and in enforcing it. Water quality criteria are usually
established for drinking water, and for surface and ground waters. The control of
the discharge of effluent has become a major preoccupation for industries in
Europe and North America. Legislation often sets maximum daily limits and a
longer-term average limit for a whole range of contaminants. Controls in
developing countries are much less stringent and, in some cases, almost non-
existent. This has been a significant factor in the recent re-structuring of the
dyestuffs manufacturing and textile finishing industries.
There is little doubt that water quality standards will become increasingly
important. Therefore, effluent treatment before discharge will be required, with
increasing costs to the textile industry. The impact of textile effluent on aquatic
life is an active field of research. There is considerable collaboration through
ETAD (the Ecological and Toxicological Association of the Dyes and Organic
Pigments Manufacturing Industry [3]) and ADMI (the American Dye
Manufacturers Institute). Both have undertaken numerous studies in this area.
Most textile effluent water is discharged into surface waters such as rivers and
lakes, either directly or through municipal sewers. The main problem is the wide
range of chemicals that it contains and the high level of dilution that usually
exists. Table 8.4 lists some of the common pollutants. Of particular concern are
those chemicals that are not degraded by water-borne bacteria, and which
therefore persist and accumulate in the environment. Many such chemicals, such
as polychlorobiphenyls, are extremely toxic and dangerous.
Discharge of effluent to a municipal sewer has the advantage that the sewage
treatment is able to remove many, but not all, of the contaminants. Provided the
nature and approximate amounts of the contaminants are known, sewage
treatment can reduce pollutant levels to the point at which discharge into surface
water is then feasible.
Because of the diversity of chemicals in textile effluent, it is usually
characterised by a number of general criteria rather than in terms of specific
contaminants. These include those described below.
(1) The volume of effluent.
(2) A measure of the amount of oxygen it will consume for oxidation of the organic chemicals it contains. This point is important because depletion of oxygen in water has a negative impact on aquatic life. The biological oxygen demand (BOD) [1] is the amount of o xygen (mg l–1 or ppm) consumed in 5 days at 20 ° C by growth of bacteria from a culture added to the water. The chemical oxygen demand (COD) [1] is based on a much faster chemical oxidation of organic compounds with hot sodium dichromate solution. The two values are often close but not equivalent. Many organic compounds are readily oxidised by hot dichromate but are resistant to microbial oxidation at ambient temperature. It is typical of textile effluent that the COD is much higher than the BOD. The COD is less affected by the usual effluent treatment processes, and is thus more persistent in the environment. Values range from 200 – 3000 mg O2 l–1 for BOD and from 500 – 5000 mg O2 l–1 for COD. The total organic carbon (TOC) in the water serves as an alternative to BOD and COD. All these can be determined by standardised analytical procedures.
(3) Floating insoluble chemicals, mainly insoluble oils and solvents.
(4) Suspended solid materials. These are quite diverse and include short fibres and insoluble dyes or compounds that have precipitated in the effluent because of a change in temperature or pH. Quantities range from 50 – 500 mg l–1. This can be estimated by filtration or by turbidity measurements.
(5) Colour. This is visible pollution. While it may not be toxic, colour does reduce light transmission into waters and limits photosynthesis. The dyeing industry discharges about 9% of the dyestuffs it consumes. This corresponds to a considerable degree of colour in a dyehouse effluent. Dyes are not easily biodegraded since, by design, they have good stability towards light and various chemical treatments. Most dyes are not of high toxicity and are eventually removed from water by oxidation or adsorption on sediment, but presence of colour in the water from a dyehouse is undesirable. It is a strong indicator of the presence of much higher quantities of dyeing assistants, almost all of which are present in the effluent. Even a simple chemical such as acetic acid can significantly increase the BOD.
(6) Acidity. The pH may vary from about 4 up to near 12. The acidity of water affects aquatic life and effluent must be neither too acidic nor too alkaline on discharge. This also applies for discharge into a municipal sewer because the micro-organisms used in sewage treatment are equally susceptible.
(7) Toxic chemicals. For the textile industry, the major offenders here are heavy metals such as chromium and copper, organochlorine compounds from insecticides or moth-proofing agents, and sulphides from dyeing with sulphur dyes. We also study about- Effluent treatment, Dye-house water treatment, Water treatment in dyeing, importance of water treatment in the dyeing, Water quality in dyeing, benefits of treated water in dyeing, different types of water treatment plant, Methods of water treatment.
(1) The volume of effluent.
(2) A measure of the amount of oxygen it will consume for oxidation of the organic chemicals it contains. This point is important because depletion of oxygen in water has a negative impact on aquatic life. The biological oxygen demand (BOD) [1] is the amount of o xygen (mg l–1 or ppm) consumed in 5 days at 20 ° C by growth of bacteria from a culture added to the water. The chemical oxygen demand (COD) [1] is based on a much faster chemical oxidation of organic compounds with hot sodium dichromate solution. The two values are often close but not equivalent. Many organic compounds are readily oxidised by hot dichromate but are resistant to microbial oxidation at ambient temperature. It is typical of textile effluent that the COD is much higher than the BOD. The COD is less affected by the usual effluent treatment processes, and is thus more persistent in the environment. Values range from 200 – 3000 mg O2 l–1 for BOD and from 500 – 5000 mg O2 l–1 for COD. The total organic carbon (TOC) in the water serves as an alternative to BOD and COD. All these can be determined by standardised analytical procedures.
(3) Floating insoluble chemicals, mainly insoluble oils and solvents.
(4) Suspended solid materials. These are quite diverse and include short fibres and insoluble dyes or compounds that have precipitated in the effluent because of a change in temperature or pH. Quantities range from 50 – 500 mg l–1. This can be estimated by filtration or by turbidity measurements.
(5) Colour. This is visible pollution. While it may not be toxic, colour does reduce light transmission into waters and limits photosynthesis. The dyeing industry discharges about 9% of the dyestuffs it consumes. This corresponds to a considerable degree of colour in a dyehouse effluent. Dyes are not easily biodegraded since, by design, they have good stability towards light and various chemical treatments. Most dyes are not of high toxicity and are eventually removed from water by oxidation or adsorption on sediment, but presence of colour in the water from a dyehouse is undesirable. It is a strong indicator of the presence of much higher quantities of dyeing assistants, almost all of which are present in the effluent. Even a simple chemical such as acetic acid can significantly increase the BOD.
(6) Acidity. The pH may vary from about 4 up to near 12. The acidity of water affects aquatic life and effluent must be neither too acidic nor too alkaline on discharge. This also applies for discharge into a municipal sewer because the micro-organisms used in sewage treatment are equally susceptible.
(7) Toxic chemicals. For the textile industry, the major offenders here are heavy metals such as chromium and copper, organochlorine compounds from insecticides or moth-proofing agents, and sulphides from dyeing with sulphur dyes. We also study about- Effluent treatment, Dye-house water treatment, Water treatment in dyeing, importance of water treatment in the dyeing, Water quality in dyeing, benefits of treated water in dyeing, different types of water treatment plant, Methods of water treatment.
BOILER WATER
Boilers for steam generation have varying operating pressures and capacities. The
use of a high pressure boiler allows a greater generation capacity but to minimise
some of the problems discussed below requires a much higher water quality than a
low pressure system.
Temporary hardness in boiler feed water gives an accumulation of chalk scum in
the boiler and scale on the walls and heating tubes. Deposition on the latter
greatly reduces the rate of heat transfer and the boiler becomes increasingly less
efficient. At high temperatures and pressures, both calcium and magnesium
carbonates and magnesium hydroxide are much less soluble than under ambient
conditions and contribute to scaling. Simple phosphates such as Na2HPO4, added
to boiler feed water, will precipitate insoluble calcium and magnesium phosphate
in a form that does not form a crust on the boiler walls and pipes. Polyphosphate
sequestrants are less helpful since they tend to hydrolyse rapidly to simple
phosphates in boiling water. Treatment with simple phosphates also ensures
absorption of colloidal silica. This is significant, since silica scale on pipes and
walls is very difficult to remove.
The precipitation of calcium and magnesium in a non-crusting form in the
boiler produces suspended material, the rate of accumulation being greater the
higher the pressure and the capacity of the boiler. It is quite common to add
dispersants such as polyacrylates to the feed water as these keep the precipitates
well dispersed to prevent scaling. Discharge of the sludge is necessary from time to
time to avoid excessive accumulation and to keep the salinity of the water within
reasonable limits. In addition, dispersants and anti-foam chemicals prevent carry-
over of sediment and foam with the generated steam.
It is vital that there is minimum corrosion of the boiler and piping. This can be
caused by acids, and by dissolved carbon dioxide and oxygen. Iron fittings corrode
rapidly if boiler feed water is too acidic, so it is usual to condition the water to
about pH 8 – 9 by addition of NaOH. This also ensures that all carbon dioxide is
converted into bicarbonate.
Too high a pH causes caustic embrittlement of non-ferrous metal fittings such
as rivets. On the other hand, at pH values below 6, carbon dioxide can attack iron
to form ferrous bicarbonate. This can occur in the boiler and in the piping for
steam distribution and condensate return. Sometimes, volatile amines, such as
ammonia or cyclohexylamine, are added to the water to neutralise any acidity and
prevent this.
Dissolved oxygen is a major source of corrosion. Preliminary heating removes
most oxygen, since this gas is much less soluble in hot water. Alternatively, it
reacts with reducing agents such as sodium sulphite or hydrazine added to the feed
water. Sulphite is oxidised to sulphate but hydrazine has the advantage that it does
not produce any ionic products.
Following matters are important in dye-house- Steam making with boiler, steam produce with boiler,The quality of steam produced from boiler, The capacity of boiler, pressure in the boiler, Mechanism of steam producing in the boiler, dye-house steam produce with boiler, End use of the boiler steam, The quality of water required to produce boiler water.
Following matters are important in dye-house- Steam making with boiler, steam produce with boiler,The quality of steam produced from boiler, The capacity of boiler, pressure in the boiler, Mechanism of steam producing in the boiler, dye-house steam produce with boiler, End use of the boiler steam, The quality of water required to produce boiler water.
Sequestering agents
Transition metal ions in the water supply can pose difficult problems in a
dyehouse. Firstly, many of these ions catalyse the decomposition of hydrogen
peroxide in bleaching baths. In addition, transition metals often give insoluble salts with dyes, or form complexes that are invariably duller and even different in
shade. If the amounts are excessive, the water may be unusable without treatment.
On adequate aeration of the water at pH values around 7, iron precipitates as
insoluble Fe(OH)3 and can be removed. There is always concern about the
possibility of iron from corrosion inside the water pipes in a textile plant.
Addition of a sequestering agent to the water avoids many problems from
relatively low concentrations of undesirable metal ions. Sequestering agents react
with the metal ions to form very stable complex ions. Examples of such chemicals
used in textile processing include EDTA, and related aminocarboxylic acids, as
well as polyphosphates such as sodium tetrametaphosphate Na4P4O12. EDTA is a
most effective sequestering agent, particularly in neutral or weakly alkaline
solution. It forms such stable complexes with metal ions that it often removes the
metal ion from a metal– complex dyestuff molecule, to give the EDTA – metal
complex and uncomplexed dye.
Polyphosphates can bind alkaline earth metal ions and thus decrease the effects
of water hardness. The product Calgon, sodium hexametaphosphate Na6P6O18, is
widely used for this purpose. Calcium ions replace sodium in the
hexametaphosphate, forming a stable complex. The free calcium ion
concentration in the water is then so low that calcium soaps do not precipitate.
Polyphosphates are frequently present in domestic washing powders for the same
reason.
Water softening with Ion exchange methods
A zeolite is a naturally occurring insoluble mineral of the sodium aluminosilicate
type. When hard water passes through a bed of small particles of such a mineral,
an ion exchange reaction takes place (Scheme 8.10). As more and more alkaline
earth metal ions are retained by the zeolite, its exchange capacity gradually
decreases. Regeneration of the sodium salt of the zeolite involves passing a
concentrated solution of NaCl through the zeolite. The calcium and magnesium
ions are displaced and leave with the solution (the reverse of Scheme 8.10). After
rinsing with water to eliminate the excess salt, the zeolite is ready for another cycle
of softening.
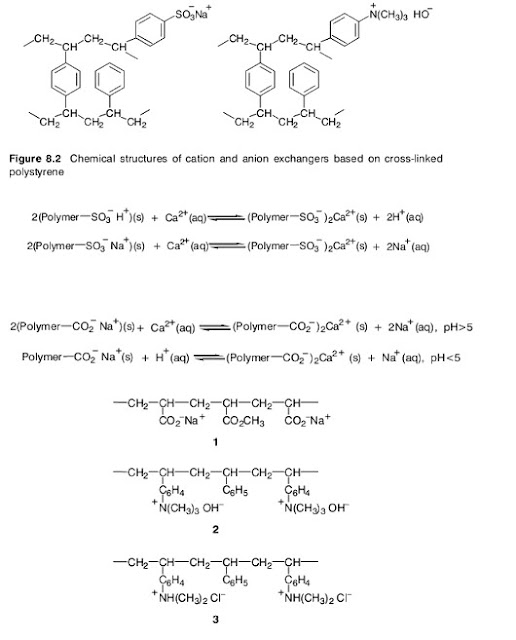
Many zeolites occur in nature but they can also be manufactured. The removal of the alkaline earth metal ions is more effective the greater the surface area of the zeolite particles in contact with the water. For this reason, softening involves percolation of the water down a packed column of the finely ground zeolite, followed by periodic regeneration. Although the calcium and magnesium ions in the water are replaced by sodium ions, these are relatively harmless in textile processing.The chemical structures of aluminosilicates are based on the structure of silica.This consists of a three dimensional network of SiO4 units, in which the oxygen atoms have a tetrahedral arrangement around the central silicon atom. These tetrahedra may have common corners or faces. In an aluminosilicate, a number of aluminium atoms replace silicon atoms in the silica structure. The aluminium atoms are bonded to four tetrahedral oxygen atoms but because their atomic number is one less than silicon, each aluminium atom introduced has a negative charge, balanced by incorporation of a cation such as Na+ or K+. It is these cations that are available for exchange. The newer synthetic polymer ion exchangers are much more versatile than the zeolites and are widely used for water softening and demineralisation. They are often called ion exchange resins. Many are based on polystyrene that has been partly crosslinked by incorporation of a small amount of divinylbenzene (2 – 10%). Suspension polymerisation of the styrene and divinylbenzene produces the crosslinked polymer in the form of small beads. These have the appearance of a fine white sand. Sulphonation of some of the benzene rings, mainly on the bead surfaces, provides anionic sites suitable for cation exchange (Figure 8.2). Since a sulphonic acid is comparable in strength to a mineral acid, these are called strong acid exchangers. They may absorb cations and release hydrogen ions, or, in the form of their sodium salts, exchange sodium ions for other cations in the water
Weak acid cation exchanger, containing acrylic acid (ethenoic acid) units ( 1 ); strong basic anion exchange resin, containing quaternary ammonium hydroxide groups (2 );weakly basic anion exchange resin with protonated secondary amino groups (3 ) The so-called weak acid cation exchangers are usually polymers containing carboxylic acid groups, such as vinyl polymers containing acrylic acid (ethenoic acid) units (1, in Figure 8.3). These are used in the form of their sodium salts. They do not exchange hydrogen ions for cations below pH 5 since the free carboxylic acid groups are barely dissociated at low pH values (Scheme 8.12). Other types of ion exchange resins are available for anion exchange. These may be of the strong basic type, containing quaternary ammonium hydroxide groups (see Figure 8.2, and 2 in Figure 8.3), or weakly basic with protonated secondary amino groups (3 , in Figure 8.3). Either type will have associated counter anions that exchange with anions in the solution in contact with the resin. The weakly basic types have ammonium ion groups that can lose a proton in contact with an alkaline solution and therefore are ineffective above pH 10 (Scheme 8.13). The ion exchange processes are again reversible so resin regeneration simply involves treatment with a concentrated solution of a salt containing the appropriate anion.
Therefore, a strongly basic quaternary ammonium ion resin is regenerated using NaOH solution, and a secondary amine type with NaCl or HCl solution. The two most important properties of an ion exchanger are its capacity and its selectivity. The capacity is the number of ions that a given mass of resin is capable of binding by exchange, normally expressed in milliequivalents per gram of dry or of wet resin. For example, 1.0 mmol of Na+ is the same as 1.0 mequiv, but 1.0 mmol of Ca2+ is 2.0 mequiv. Therefore, a resin with a capacity of 15.0 mequiv g– 1 would be capable of binding 15.0 mmol g– 1 Na+ or 7.5 mmol g–1 Ca2+. The selectivity of the resin determines how strongly it binds a given ion and therefore its ease of exchange. Clearly, for water softening a cation exchange resin should have a higher selectivity for Ca2+ and Mg2+ than for H+ or Na+. Fortunately, the selectivity is often greater for ions of higher ionic charge. Besides these two properties, the degree of swelling of the resin in contact with the water must be limited. The higher the degree of crosslinking, for example from incorporation of more divinylbenzene in the polystyrene, the lower the extent of swelling. It is also important that the resin particles have a large surface area and that water is able to penetrate into the surface pores. Water can be totally demineralised by firstly exchanging all cations using a strongly acid form of a cation exchanger. Thus, a solution of salts M+X– becomes a solution of acid H+X–, the M+ ions being retained by the resin. Subsequent percolation through a packing of a strongly basic form of an anion exchanger absorbs the X– ions and liberates HO– ions into the water. These then neutralise the H+ ions from the first step. The result is retention of all anions and cations and the neutralisation of H+ and HO– to form water (Scheme 8.14). Thus, the
water has been demineralised. It may, however, still contain organic material and dissolved carbon dioxide from the reaction of carbonate and bicarbonate with the acid from the resin. A thorough aeration eliminates the carbon dioxide. Demineralisation is important for water fed to very high pressure boilers. The use of ion exchange resins for water treatment is relatively simple. The resin is packed into a column containing water and treatment simply involves flowing water up or down the column. The capacity of the resin and the ionic content of the water determine when regeneration will be required. One problem with beds of ion exchangers is the retention in the column of suspended matter and living organisms in the water. Countercurrent rinsing and occasional treatment with a bactericide minimise these problems. For removal of both cations and anions (demineralisation), two columns in series are used, the first for strong acid exchange and the second for strong base exchange. It is even possible to mix anion and cation exchangers in the same bed. If the different types of particles have different densities, they can be separated by sedimentation in a counterflow of water, regenerated separately, and then re-mixed. Figure 8.4 shows a typical series of processes for water softening.
Many zeolites occur in nature but they can also be manufactured. The removal of the alkaline earth metal ions is more effective the greater the surface area of the zeolite particles in contact with the water. For this reason, softening involves percolation of the water down a packed column of the finely ground zeolite, followed by periodic regeneration. Although the calcium and magnesium ions in the water are replaced by sodium ions, these are relatively harmless in textile processing.The chemical structures of aluminosilicates are based on the structure of silica.This consists of a three dimensional network of SiO4 units, in which the oxygen atoms have a tetrahedral arrangement around the central silicon atom. These tetrahedra may have common corners or faces. In an aluminosilicate, a number of aluminium atoms replace silicon atoms in the silica structure. The aluminium atoms are bonded to four tetrahedral oxygen atoms but because their atomic number is one less than silicon, each aluminium atom introduced has a negative charge, balanced by incorporation of a cation such as Na+ or K+. It is these cations that are available for exchange. The newer synthetic polymer ion exchangers are much more versatile than the zeolites and are widely used for water softening and demineralisation. They are often called ion exchange resins. Many are based on polystyrene that has been partly crosslinked by incorporation of a small amount of divinylbenzene (2 – 10%). Suspension polymerisation of the styrene and divinylbenzene produces the crosslinked polymer in the form of small beads. These have the appearance of a fine white sand. Sulphonation of some of the benzene rings, mainly on the bead surfaces, provides anionic sites suitable for cation exchange (Figure 8.2). Since a sulphonic acid is comparable in strength to a mineral acid, these are called strong acid exchangers. They may absorb cations and release hydrogen ions, or, in the form of their sodium salts, exchange sodium ions for other cations in the water
Weak acid cation exchanger, containing acrylic acid (ethenoic acid) units ( 1 ); strong basic anion exchange resin, containing quaternary ammonium hydroxide groups (2 );weakly basic anion exchange resin with protonated secondary amino groups (3 ) The so-called weak acid cation exchangers are usually polymers containing carboxylic acid groups, such as vinyl polymers containing acrylic acid (ethenoic acid) units (1, in Figure 8.3). These are used in the form of their sodium salts. They do not exchange hydrogen ions for cations below pH 5 since the free carboxylic acid groups are barely dissociated at low pH values (Scheme 8.12). Other types of ion exchange resins are available for anion exchange. These may be of the strong basic type, containing quaternary ammonium hydroxide groups (see Figure 8.2, and 2 in Figure 8.3), or weakly basic with protonated secondary amino groups (3 , in Figure 8.3). Either type will have associated counter anions that exchange with anions in the solution in contact with the resin. The weakly basic types have ammonium ion groups that can lose a proton in contact with an alkaline solution and therefore are ineffective above pH 10 (Scheme 8.13). The ion exchange processes are again reversible so resin regeneration simply involves treatment with a concentrated solution of a salt containing the appropriate anion.
Therefore, a strongly basic quaternary ammonium ion resin is regenerated using NaOH solution, and a secondary amine type with NaCl or HCl solution. The two most important properties of an ion exchanger are its capacity and its selectivity. The capacity is the number of ions that a given mass of resin is capable of binding by exchange, normally expressed in milliequivalents per gram of dry or of wet resin. For example, 1.0 mmol of Na+ is the same as 1.0 mequiv, but 1.0 mmol of Ca2+ is 2.0 mequiv. Therefore, a resin with a capacity of 15.0 mequiv g– 1 would be capable of binding 15.0 mmol g– 1 Na+ or 7.5 mmol g–1 Ca2+. The selectivity of the resin determines how strongly it binds a given ion and therefore its ease of exchange. Clearly, for water softening a cation exchange resin should have a higher selectivity for Ca2+ and Mg2+ than for H+ or Na+. Fortunately, the selectivity is often greater for ions of higher ionic charge. Besides these two properties, the degree of swelling of the resin in contact with the water must be limited. The higher the degree of crosslinking, for example from incorporation of more divinylbenzene in the polystyrene, the lower the extent of swelling. It is also important that the resin particles have a large surface area and that water is able to penetrate into the surface pores. Water can be totally demineralised by firstly exchanging all cations using a strongly acid form of a cation exchanger. Thus, a solution of salts M+X– becomes a solution of acid H+X–, the M+ ions being retained by the resin. Subsequent percolation through a packing of a strongly basic form of an anion exchanger absorbs the X– ions and liberates HO– ions into the water. These then neutralise the H+ ions from the first step. The result is retention of all anions and cations and the neutralisation of H+ and HO– to form water (Scheme 8.14). Thus, the
water has been demineralised. It may, however, still contain organic material and dissolved carbon dioxide from the reaction of carbonate and bicarbonate with the acid from the resin. A thorough aeration eliminates the carbon dioxide. Demineralisation is important for water fed to very high pressure boilers. The use of ion exchange resins for water treatment is relatively simple. The resin is packed into a column containing water and treatment simply involves flowing water up or down the column. The capacity of the resin and the ionic content of the water determine when regeneration will be required. One problem with beds of ion exchangers is the retention in the column of suspended matter and living organisms in the water. Countercurrent rinsing and occasional treatment with a bactericide minimise these problems. For removal of both cations and anions (demineralisation), two columns in series are used, the first for strong acid exchange and the second for strong base exchange. It is even possible to mix anion and cation exchangers in the same bed. If the different types of particles have different densities, they can be separated by sedimentation in a counterflow of water, regenerated separately, and then re-mixed. Figure 8.4 shows a typical series of processes for water softening.
Water Softening with lime-soda process
The old lime-soda process is now obsolete but was very useful for the treatment of
large volumes of hard water. Addition of lime (CaO) and soda (Na2CO3) to the
hard water precipitates calcium as the carbonate, and magnesium as its hydroxide.
The amounts of the two chemicals required are easily calculated from the analysis
of the water and stoichiometry of the reactions (Scheme 8.9). Since calcium
carbonate and magnesium hydroxide are not completely insoluble, the water obtained has a residual hardness of not less than 20 ppm CaCO3. A major problem of this type of process is the disposal of the sludge of precipitated calcium carbonate and magnesium hydroxide.
carbonate and magnesium hydroxide are not completely insoluble, the water obtained has a residual hardness of not less than 20 ppm CaCO3. A major problem of this type of process is the disposal of the sludge of precipitated calcium carbonate and magnesium hydroxide.
WATER SOFTENING
Soft water is a desirable prerequisite for all textile wet processes, except for
bleaching with solutions of hydrogen peroxide stabilised by sodium silicate.
Modern synthetic detergents do not form precipitates in hard water containing
calcium and magnesium ions so a certain degree of hardness is tolerable provided
that other dyes and chemicals are not seriously affected by this. In general,
however, if a mill is in a region where the water is hard, a softening pretreatment is
essential for at least part of the water used. The objective of this is simply to
reduce the concentration of the alkaline earth metals to a level at which the water
has the desired quality. In many cases, the softening process may reduce the
calcium and magnesium ion concentrations to zero.
Water alkalinity
Various dissolved salts in natural water give it an alkaline pH. These are mainly
carbonates, bicarbonates and phosphates (Scheme 8.7).
The alkalinity of a water sample can be determined by titration with standardised sulphuric acid solution. With phenolphthalein as the indicator, the
equivalence point occurs at about pH 8.3. At this point, all free hydroxide ion is
neutralised, carbonate converted into bicarbonate, and phosphate (PO43–) into monohydrogen phosphate (HPO42–). The alkalinity to pH 8.3 is calculated in ppm CaCO3 based on Scheme 8.8. Therefore, each millimole of sulphuric acid required corresponds to 1.00 mmol CaCO3 in the water sample. If the titration of a 100.0 ml water sample with 0.01 M sulphuric acid consumes V ml of acid, the alkalinity is given by:
At the phenolphthalein end-point of pH 8.3, the water is still slightly alkaline and it is therefore common practice to titrate the sample to a methyl orange end-point, corresponding to about pH 4.5. This ensures that all carbonate is in the from of carbonic acid and all phosphate is present as dihydrogenphosphate (H2PO4–). The alkalinity to pH 4.5, often called the total alkalinity, is given by the same equation as above, but, of course, the volume of sulphuric acid solution may be greater.
Table 8.3 relates the total (T) alkalinity and phenolphthalein alkalinity (P) of various types of water to the dissolved salts that are present. In the absence of phosphate ions, there will be some correlation between the alkalinity caused by bicarbonate and the temporary hardness. For example, if the alkalinity to
phenolphthalein is zero (P = 0), the total alkalinity, assuming no phosphates or weakly acidic pollutants, will be caused by bicarbonate ion and the total alkalinity in ppm CaCO3 will equal the temporary hardness. The alkalinity of water fed to boilers must not be too high because of the risk of corrosion of non-ferrous fittings.
At the phenolphthalein end-point of pH 8.3, the water is still slightly alkaline and it is therefore common practice to titrate the sample to a methyl orange end-point, corresponding to about pH 4.5. This ensures that all carbonate is in the from of carbonic acid and all phosphate is present as dihydrogenphosphate (H2PO4–). The alkalinity to pH 4.5, often called the total alkalinity, is given by the same equation as above, but, of course, the volume of sulphuric acid solution may be greater.
Table 8.3 relates the total (T) alkalinity and phenolphthalein alkalinity (P) of various types of water to the dissolved salts that are present. In the absence of phosphate ions, there will be some correlation between the alkalinity caused by bicarbonate and the temporary hardness. For example, if the alkalinity to
phenolphthalein is zero (P = 0), the total alkalinity, assuming no phosphates or weakly acidic pollutants, will be caused by bicarbonate ion and the total alkalinity in ppm CaCO3 will equal the temporary hardness. The alkalinity of water fed to boilers must not be too high because of the risk of corrosion of non-ferrous fittings.
Subscribe to:
Posts (Atom)